Toxicopathological Effect Of Benzene, Toluene, Ethylbenzene, And Xylenes (BTEX) As A Mixture And The Protective Effect Of Citicoline In Male Rats Followings 90-Day Oral Exposure
Abstract
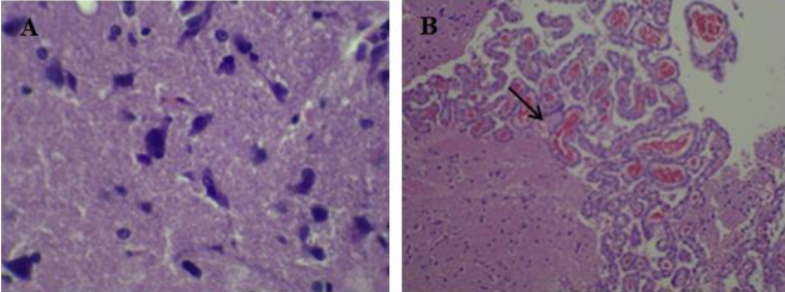
The chemicals benzene, toluene, ethylbenzene, and xylene (BTEX) are neurotoxic. Although health effects of the individual (BTEX) chemicals are generally well described, relatively less is known on the toxicity following the exposures to “whole” mixtures. In the present study, the chronic toxicity of BTEX mixture following 90 days of consecutive oral exposure and the protective effect of citicoline was investigated in male rats. Doses for the BTEX mixture were selected based on the no observed adverse effect level NOAEL, as determined in previous animal toxicity studies for each compound. A total of 64 rats were divided in four equal groups of 16 male rats each. Rats in G1were the control. Rats in G2 were gavaged with BTEX mixture at doses of 600 mg/kg. Rats in G3 were exposed to BTEX mixture at 600 mg/kg in combination with citicoline 500 mg/kg orally. Rats in G4 were treated with citicoline at 500 mg/kg only. All treatments were conducted 7 days/per week for 90-day oral exposure. Histopathological changes in the brain, liver and kidney and caspase-3-activity in brain stained slides were evaluated in this study. Rats treated with BTEX showed histopathological alteration such as microgliosis in the brain with sever degenerative changes in the liver and kidney and marked increase of caspase-3 activity in the brain. Rats treated with BTEX and citicoline showed improvement in brain, liver architecture and kidney and marked decrease in the brain positive caspase-3 activity.
In conclusion, the results showed that the safe level (NOAEL) of BTEX compound individually, can produce a significant adverse effect when administrated as a mixture in male rats. The results also indicated that citicoline treatment can protect against BTEX induced toxicity in male rats.